Relative humidity (RH) mediates a number of mechanisms through which viral infections such as COVID-19 can increase fatality rates during the fall/weather season.
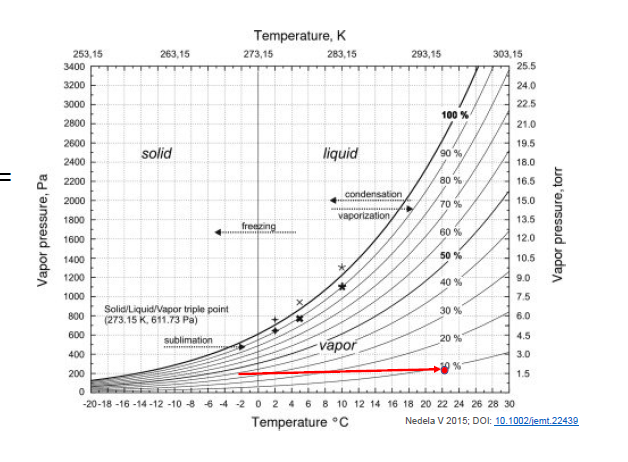
The night before last here in Boston (posted November 27, 2020), the temp dropped to 28 deg F (-2.2 C). The outside RH was ~ 40%. Meanwhile, the indoor temp is ~ 70 deg F (21 C), this translates to an indoor RH of 10%.
What happens then?
First, the lower RH can impair mucociliary clearance – as beautifully demonstrated by Professor Akiko Iwasaki @virusesimmunity. (https://twitter.com/VirusesImmunity/status/1320430656481120256)
As we approach the cold winter months in the Northern Hemisphere, I want to share these movies of mucociliary clearance (MCC) in the trachea of mice housed at 10% vs. 50% relative humidity (RH). Captured by @ericsongg (1/n)
This is MCC at 50% RH 👇🏽 pic.twitter.com/te5UKxIz4h
— Prof. Akiko Iwasaki (@VirusesImmunity) October 25, 2020
Second, the lower RH can increase viral viability (via efflorescence) as shown in the insightful mechanistic study by @dylanhmorris , @linseymarr: https://twitter.com/dylanhmorris/status/1317452511624790017
Third, the lower RH can substantially increase droplet evaporation and thus sustain longer suspension of airborne droplets. This thread will focus on this factor – specifically the physics – and evaluate how big a factor this may play.
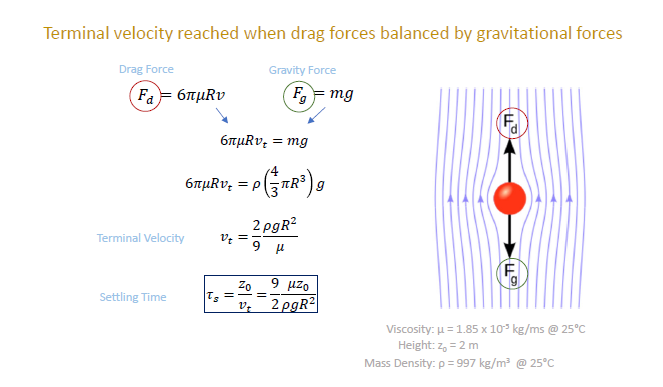
To start, how long does it take for a droplet (with radius, R) to fall to the ground? Terminal velocity is reached when drag forces is balanced by gravitational force. (NOTE: vt is reached quickly. Just 0.12 secs even for large inertial 100 um droplet).
This graph shows settling time w.r.t. droplet radius:
1 um => 4.7 hrs
50 um => 6.8 sec
100 um => 1.7 secs.
But how can this be? Didn’t aerosol scientists say that even 100 um droplets remain in air for a long time?
We haven’t considered evaporation yet. Math here gets more complicated, but ultimately time for water droplet to fully evaporate is given by the following formula. (Obtained from Netz RR 2020 pubs.acs.org/doi/abs/10.102) NOTE: RH is key. Not absolute humidity.
Evaporation time is graphed against Initial droplet radius by RH. Lower RH => shorter evaporation time.
On left side: settling time >> evap time (droplets fully dry up before hitting ground).
On right side: evap time >> settling time (droplets hit floor before being evaporated)
In reality, droplet size is not static as it settles: the droplet falls, it evaporates, becomes smaller, reduces the terminal velocity and then increases settling time.
Mathematically, it’s possible to account for these dynamic changes and predict the settling time by RH and height (again, not possible w/o Netz RR’s derivations). Note for RH 0.1, all droplets smaller than 77 um radius fully evaporate before hitting the ground.
Going a step further, respiratory droplets realistically don’t FULLY evaporate. Two effects lead to slow evaporation:
(1) Cooling from evaporating water molecules reduces evaporation and
(2) Solutes increases in concentration leading to osmotic retention of water.
Through some sophisticated mathematics, the following graph is obtained. We see the settling time increase with reduced radius, but does not completely evaporate into thin air. Note the sedimentation time for different radius at RH = 0.1.
Now that we got a decent grasp for settling time for the respiratory droplets, can we combine this with actual data related to droplet production from speaking and coughing? I took data from Johnson GR et al 2001 publication. sciencedirect.com/science/articl
This study identified 3 modes of aerosol production.
Bronchiolar: fluid films burst during re-expansion of small bronchiolar airways (small droplets)
Laryngeal: turbulent shearing of fluid along laryngeal walls (medium)
Oral: from coughing, speech articulation (large)
What’s neat is that Johnson et al were able to break down the release of droplets based on these 3 modes and fit to real data. As shown in these log-log plots (Left). Linear plot was included here (Right).
Now combine these Models: (1) total droplet suspension time and (2) droplet production, each w.r.t. droplet radius – and estimate cumulative virus in air after 1 hr. Speaking generates more viruses in small diameter droplets b/c it is continuous here, while coughing 1x/min.
Here we look at steady-state, viral concentration in air (also accounting for viral inactivation, ventilation, and droplet sedimentation). Note: large amounts of virion come from 40 – 55 um droplets – small enough to remain in air, but big enough to house many virions.
If we humidify the room and increase RH from 0.1 to 0.6, the amount of viruses suspended in air (and thus potentially inhaled) decreases dramatically! As much as 2.5 times less in this scenario.
Now compare to increasing ventilation: air exchange from 0 to 6. Notice that increased ventilation does not do much for these medium sized droplets (decrease by 1.4 X), but does a very good job for smaller droplets. Why?
It has to do with time constants: Greatest effect on aerosolized viral # depends on what has highest turnover (low time constant). Small droplets => ventilation Medium droplets => sedimentation (affected by RH) Larger droplets => sedimentation, but too big for any RH effect.
Optimal strategy for reducing infection can be designed accordingly.
In rooms where people are quietly breathing, sleeping (small droplets), ventilation is best.
In rooms w/ dry air, people speaking (winter holiday gatherings), humidification is best.
in this cold holiday season where people gather indoors, humidification may be the most effective course of action. Please consider humidifying your home to RH 0.4-0.6. Could be life-saving.











Great article! I really appreciate the clear and detailed insights you’ve provided on this topic. It’s always refreshing to read content that breaks things down so well, making it easy for readers to grasp even complex ideas. I also found the practical tips you’ve shared to be very helpful. Looking forward to more informative posts like this! Keep up the good work!